EFFICACY
Rapid results that LAST1*
See how ORACEA® (doxycycline, USP) 40 mg† Capsules may produce rapid results, helping treat papulopustular rosacea in patients of all body sizes.1,2 We have also included recently published data, showing how patients can maintain clearer skin through long-term use‡ of ORACEA Capsules3
Powerful results for a clearer difference you can see
Pivotal data
A non-antibiotic dose that does not
compromise on strength1
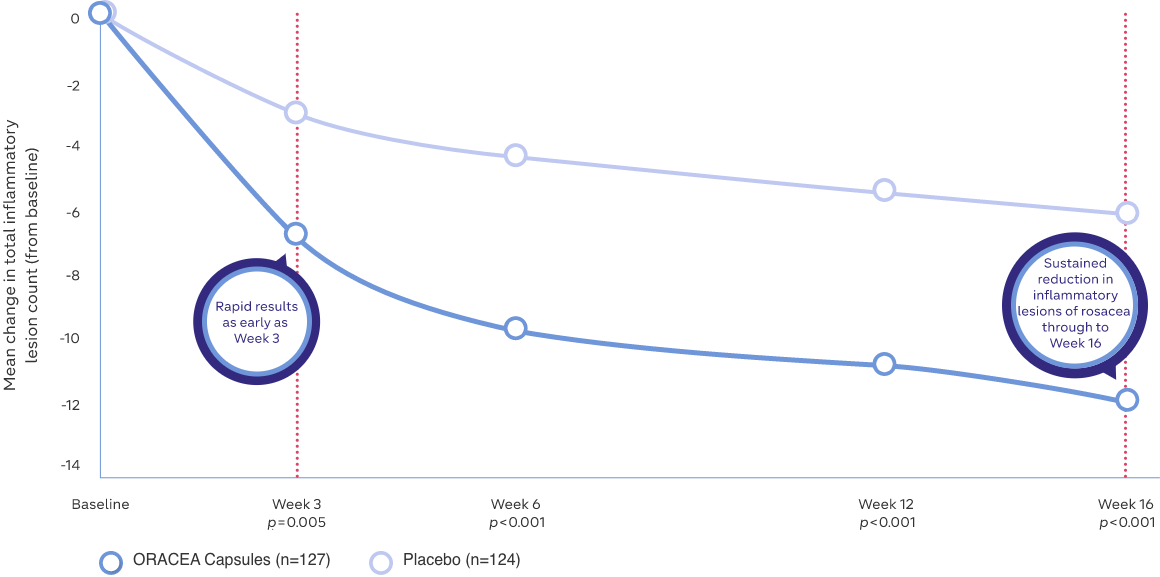
- Rapid results with clearer skin in just 3 weeks1
- Sustained reduction in inflammatory lesions by 16 weeks1
- Powerful results for your papulopustular rosacea patients1
The most common adverse events (>2%) were nasopharyngitis, sinusitis, diarrhea, hypertension and aspartate aminotransferase increase1
Study Design: A multicenter, outpatient, double-blind, placebo-controlled, parallel group trial was conducted over 16 weeks to evaluate the safety and efficacy of ORACEA Capsules. A total of 251 rosacea subjects (≥18 years of age with 10 to 40 papules and pustules and 2 nodules, plus an IGA score of 2 to 4) participated1
Adapted from: Del Rosso JQ, et al. Two randomized phase Ill clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802
*A sustained reduction in the inflammatory lesions of rosacea has been observed through to 16 weeks, in a multicenter, outpatient, double-blind, placebo-controlled, parallel group trial1
†30 mg immediate release and 10 mg delayed release beads
‡The efficacy and safety of ORACEA Capsules has been established in a 12-month clinical trial3
DOXYCYCLINE DATA
ORACEA CAPSULES OFFER POWERFUL EFFECTS
AT LESS THAN HALF THE DOSE1,4
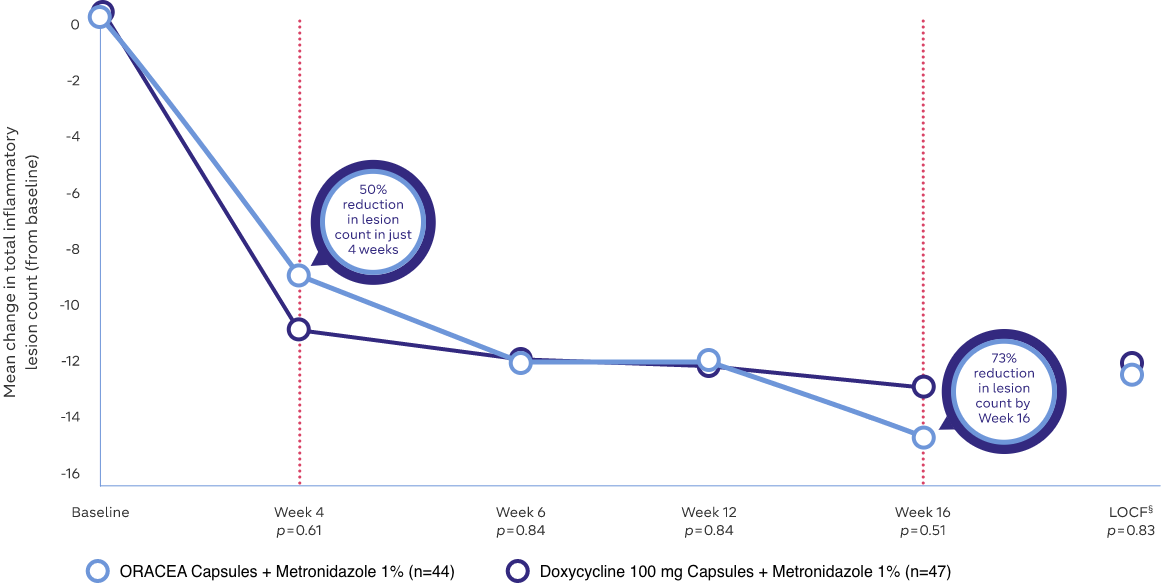
- Comparable with doxycycline 100 mg4
- 50% reduction in inflammatory lesion count in just 4 weeks4
- 73% reduction in inflammatory lesion count by Week 164
The most common adverse events (>2%) were nasopharyngitis, sinusitis, diarrhea, hypertension and aspartate aminotransferase increase1
Study Design: A randomized, multicenter, outpatient, double-blind, active-controlled, non-inferiority trial of 91 rosacea subjects (≥18 years of age) for 16 weeks. Patients were prospectively randomized to receive daily doses of either ORACEA Capsules or doxycycline (100 mg), each in combination with metronidazole cream, 1%4
Adapted from: Del Rosso JQ, et al. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7(8):573–576
§LOCF, Last observation carried forward
RELAPSE DATA
Maintaining clearer skin with ORACEA Capsules
- Continued use of ORACEA Capsules as a monotherapy for an additional 40 weeks
significantly reduced relapse rates vs placebo3 - A 50% reduction in the likelihood of relapse was seen with ORACEA Capsules from
Week 28 through to the end of the study vs placebo (27.7% vs 13.8%, p<0.05)3
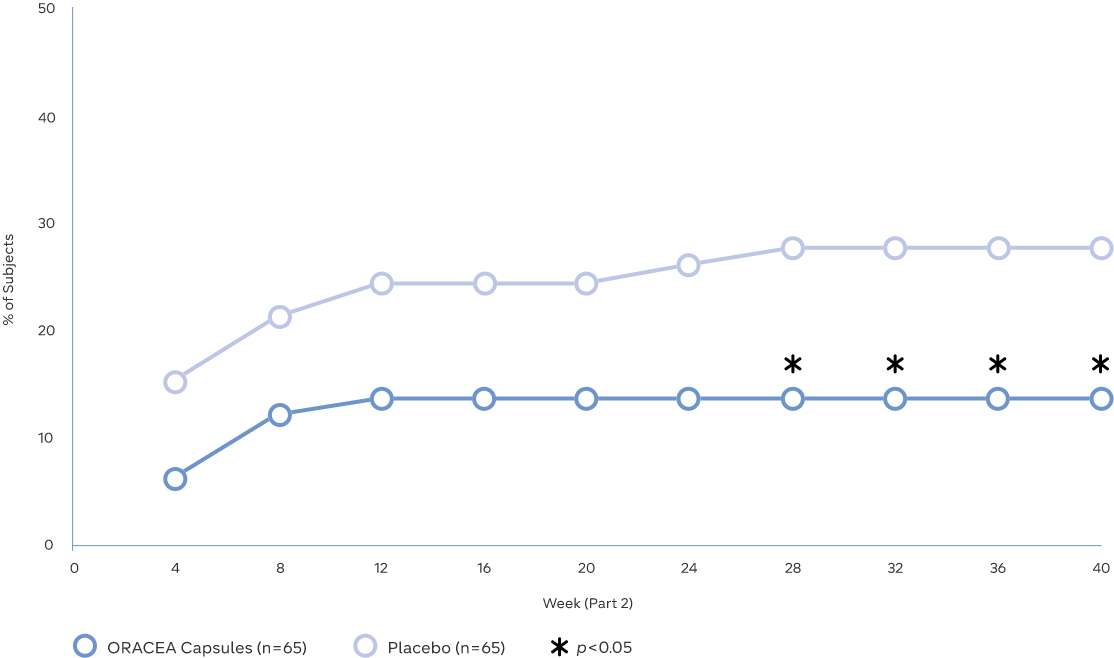
Study Design: A two-part study assessing relapse, efficacy and safety of ORACEA Capsules in patients with moderate or severe rosacea over 52 weeks (n=235). Part 1 was a 12-week multicenter, open-label study in which patients were treated with ORACEA Capsules and metronidazole gel, 1% once daily. Part 2 was a 40-week multicenter, randomized, double-blind, placebo-controlled study in which patients who achieved treatment success in Part 1 were eligible to be randomized to receive either ORACEA Capsules or placebo once daily for up to an additional 40 weeks. Subjects who relapsed during Part 2 were discontinued. The data points in the graph for Part 1 were calculated retrospectively after the patients who moved to Part 2 were randomized. Relapse was defined as a return to baseline Investigator Global Assessment (IGA) or lesion count, or any other necessary change in treatment3
Adapted from: Data on file GLI.04.SPR.US108183
Inflammatory lesions: Median change from baseline3
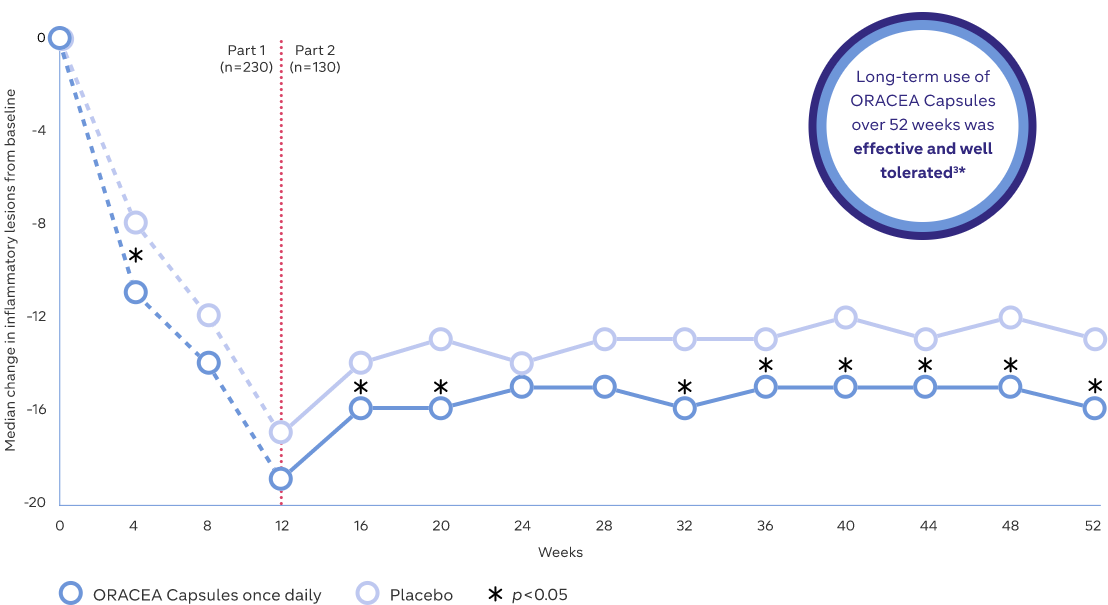
Study Design: A two-part study assessing relapse, efficacy and safety of ORACEA Capsules in patients with moderate or severe rosacea over 52 weeks (n=235). Part 1 was a 12-week multicenter, open-label study in which patients were treated with ORACEA Capsules and metronidazole gel, 1% once daily. Part 2 was a 40-week multicenter, randomized, double-blind, placebo-controlled study in which patients who achieved treatment success in Part I were eligible to be randomized to receive either ORACEA Capsules or placebo once daily for up to an additional 40 weeks. Subjects who relapsed during Part 2 were discontinued. The data points in the graph for Part 1 were calculated retrospectively after the patients who moved to Part 2 were randomized. Relapse was defined as a return to baseline Investigator Global Assessment (IGA) or lesion count, or any other necessary change in treatment3
*Both treatments were safe and well tolerated in this study. Adverse events (AEs) were generally mild or moderate in severity during both parts of the study. A similar number of AEs were reported in both the ORACEA Capsules and placebo groups (12 subjects in each group), and no serious or gastrointestinal AEs were considered to be treatment-related3

ORACEA Capsules are just as effective as doxycycline 100 mg, with fewer adverse events4
BMI RANGES AND LESION SEVERITIES
No need for weight-based dosing2
- Sustained reduced inflammation in patients with all body sizes and for all baseline lesion severities2
- With researchers beginning to unravel a link between rosacea and obesity, a treatment that
successfully reduces lesion count across all BMI may become increasingly important8
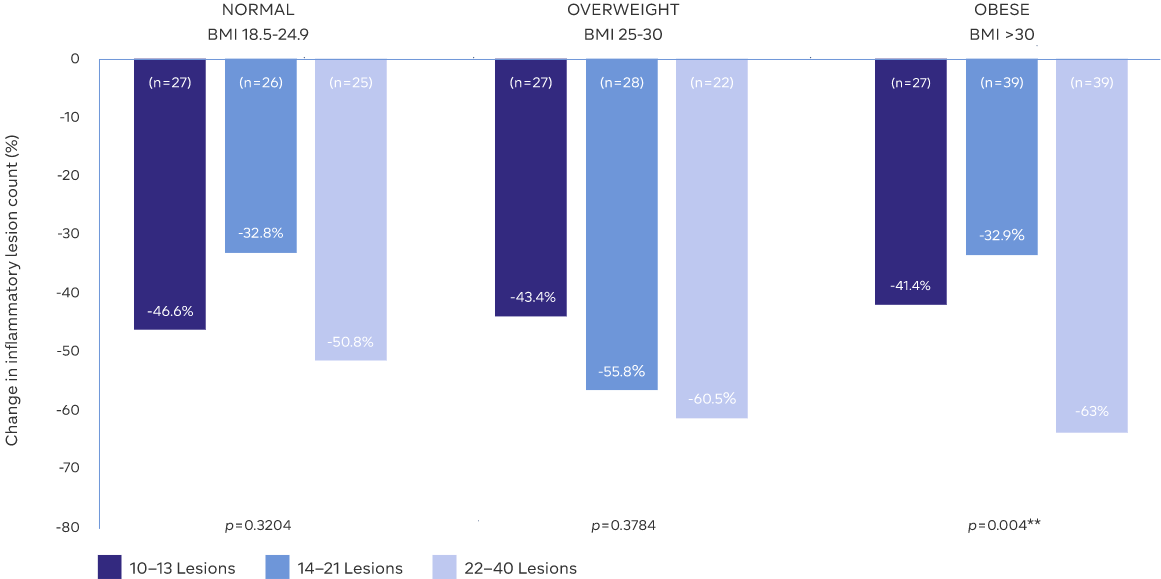
Study Design: A retrospective meta-analysis of subjects in the intent-to-treat populations from two phase 3 studies was conducted to examine if the weight of subjects and the number of baseline lesions affect the efficacy of ORACEA Capsules2
Adapted from: Data on File. GLI.04.SRE.US10148 w ADD02 CSR
**Statistically significant difference in mean percentage change of inflammatory lesion count for obese subjects2
STAY CONNECTED
Sign up and
stay up-to-date
For the latest news, research, and insights into papulopustular rosacea, subscribe now! You’ll also receive patient resources and toolkits, helping you treat your patients throughout their rosacea journey
Important Safety Information
Indication: ORACEA® (doxycycline) 40 mg* capsules are indicated for the treatment of only inflammatory lesions (papules and pustules) of rosacea in adult patients. ORACEA does not lessen the facial redness caused by rosacea. Adverse Events: In controlled clinical studies, the most commonly reported adverse events (>2%) in subjects treated with ORACEA were nasopharyngitis, diarrhea, hypertension and sinusitis. Warnings/Precautions: ORACEA should not be used to treat or prevent infections. ORACEA should not be taken by patients who have a known hypersensitivity to doxycycline or other tetracyclines. ORACEA should not be taken during pregnancy, by nursing mothers, or during tooth development (up to the age of 8 years) and may cause reversible inhibition of bone growth. If Clostridium difficile associated diarrhea (CDAD) occurs, may need to discontinue ORACEA. Although photosensitivity was not observed in clinical trials, ORACEA patients should minimize or avoid exposure to natural or artificial sunlight. The efficacy of ORACEA treatment beyond 16 weeks and safety beyond 9 months have not been established.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
*30 mg immediate release and 10 mg delayed release beads
REFERENCES
1. Del Rosso JQ, et al. Two randomized phase Ill clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. 2. Data on File. GLI.04.SRE.US10148 w ADD02 CSR. 3. Data on File. GLI.04.SPR.US10183 4. Del Rosso JQ, et al. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7(8):573–576. 5. ORACEA [package insert]. Fort Worth, TX: Galderma Laboratories, L.P.; 2014. 6. Fowler JF. Anti-inflammatory dose doxycycline for the treatment of rosacea. Expert Rev Dermatol. 2007;2(5):523–531. 7. Baldwin HE. Diagnosis and treatment of rosacea: state of the art. J Drugs Dermatol. 2012;11(6):725–730. 8. Searle, T, Al‐Niaimi, F, & Ali FR. Rosacea and the cardiovascular system. Journal of Cosmetic Dermatology. 2020;19(9): 2182-2187




