CLINICAL PHOTOS
Treatment in action:
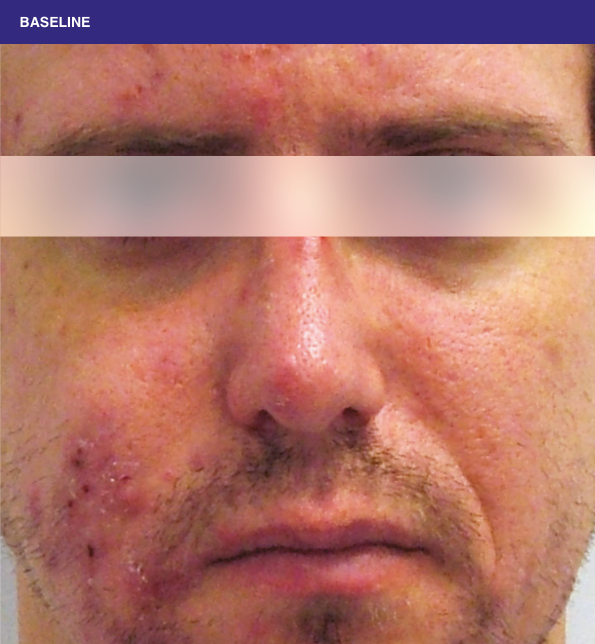
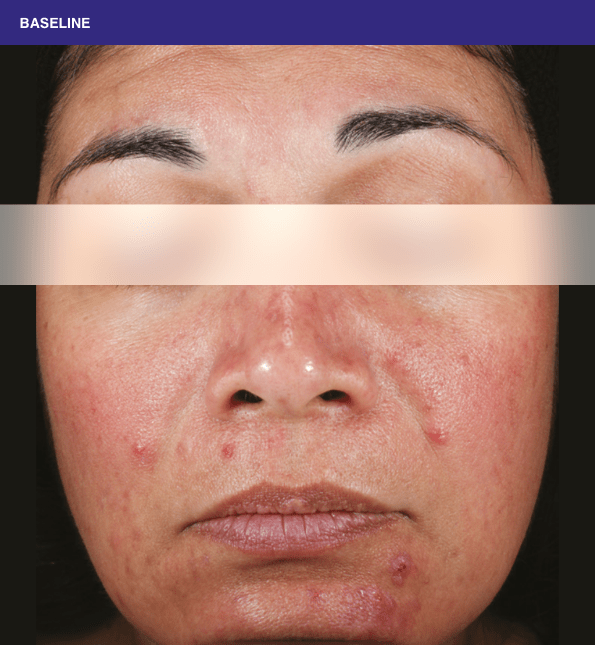
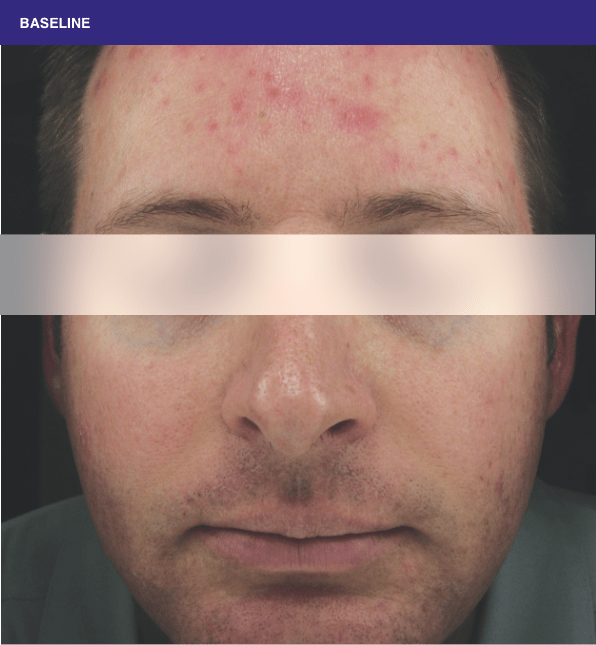
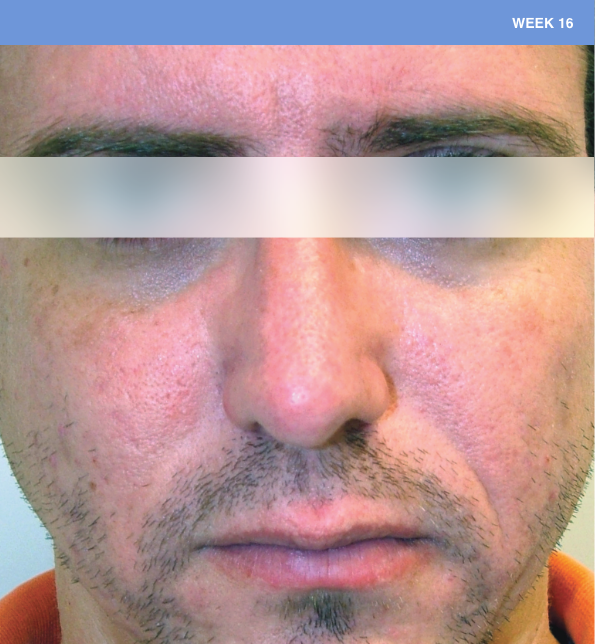
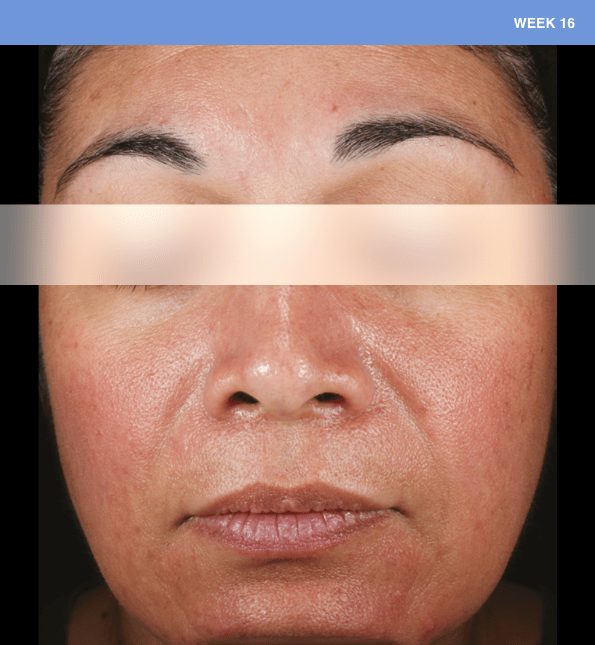
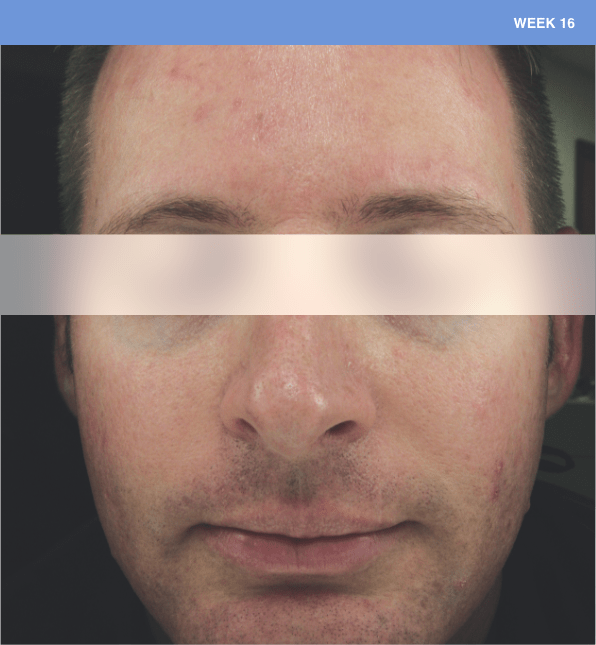
Before and after
With ORACEA® (doxycycline, USP) 40 mg* Capsules, you can offer your papulopustular rosacea patients an effective alternative to immediate-release doxycycline, without compromising on your ability to treat and get results1,2
*30 mg immediate release and 10 mg delayed release beads
†A multicenter, randomized, outpatient, double-blind, placebo-controlled, parallel group trial was conducted over 16 weeks to evaluate the safety and efficacy of ORACEA Capsules. A total of 251 subjects (≥18 years of age with 10 to 40 papules and pustules and ≥2 nodules, plus an Investigator Global Assessment [IGA] score of 2 to 4) participated3
STAY CONNECTED
Sign up and
stay up-to-date
For the latest news, research, and insights into papulopustular rosacea, subscribe now! You’ll also receive patient resources and toolkits, helping you treat your patients throughout their rosacea journey
Important Safety Information
Indication: ORACEA® (doxycycline) 40 mg* capsules are indicated for the treatment of only inflammatory lesions (papules and pustules) of rosacea in adult patients. ORACEA does not lessen the facial redness caused by rosacea. Adverse Events: In controlled clinical studies, the most commonly reported adverse events (>2%) in subjects treated with ORACEA were nasopharyngitis, diarrhea, hypertension and sinusitis. Warnings/Precautions: ORACEA should not be used to treat or prevent infections. ORACEA should not be taken by patients who have a known hypersensitivity to doxycycline or other tetracyclines. ORACEA should not be taken during pregnancy, by nursing mothers, or during tooth development (up to the age of 8 years) and may cause reversible inhibition of bone growth. If Clostridium difficile associated diarrhea (CDAD) occurs, may need to discontinue ORACEA. Although photosensitivity was not observed in clinical trials, ORACEA patients should minimize or avoid exposure to natural or artificial sunlight. The efficacy of ORACEA treatment beyond 16 weeks and safety beyond 9 months have not been established.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
*30 mg immediate release and 10 mg delayed release beads
REFERENCES
1. Del Rosso JQ, et al. Two randomized phase Ill clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. 2. Del Rosso JQ, et al. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7(8):573–576. 3. Data on File. COL-101-ROSE-301 CSR. 4. Data on File. Subject 017. 5. Baldwin HE. A community-based study of the effectiveness of doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads) on quality of life and satisfaction with treatment in participants with rosacea. Cutis. 2010;86(suppl 5[I]):26–36