ORACEA CAPSULES
Powerful and safe, at the right dose1–6
ORACEA® (doxycycline, USP) 40 mg* Capsules is the only FDA-approved oral, once-daily treatment for your papulopustular rosacea patients. It is scientifically designed to deliver a timed-release dose of doxycycline that safely keeps patients below the antibiotic threshold while providing sustained anti-inflammatory effects throughout the day3–8
Setting a standard of care in how you treat your patients has never been easier. Why compromise?
*30 mg immediate release and 10 mg delayed release beads
PIVOTAL DATA
Pivotal data backed by robust clinical results1
Clinical results showed significantly clearer skin with ORACEA Capsules1
| STUDY 1 | STUDY 2 | |||
|---|---|---|---|---|
| ORACEA Capsules (40 mg*) n=127 |
Placebo n=124 |
ORACEA Capsules (40 mg*) n=142 |
Placebo n=144 |
|
| Mean change in lesion count from baseline to Week 16 |
- 11.8 | -5.9 | -9.5 | -4.3 |
| No. (%) of subjects clear or almost clear on the IGA† |
39 (30.7%) | 24 (19.4%) | 21 (14.8%) | 9 (6.3%) |
| STUDY 1 | ||
|---|---|---|
| ORACEA Capsules (40 mg*) n=127 |
Placebo n=124 |
|
| Mean change in lesion count from baseline to Week 16 |
- 11.8 | -5.9 |
| No. (%) of subjects clear or almost clear on the IGA† |
39 (30.7%) | 24 (19.4%) |
| STUDY 2 | ||
|---|---|---|
| ORACEA Capsules (40 mg*) n=142 |
Placebo n=144 |
|
| Mean change in lesion count from baseline to Week 16 |
-9.5 | -4.3 |
| No. (%) of subjects clear or almost clear on the IGA† |
21 (14.8%) | 9 (6.3%) |
Most common adverse events in both studies (%)
| ORACEA Capsules n=269 |
Placebo n=268 |
|
|---|---|---|
| Nasopharyngitis | 4.8 % | 3.3 % |
| Diarrhea | 4.4 % | 2.6 % |
| Hypertension | 2.9 % | 0.7 % |
| Sinusitis | 2.6 % | 0.7 % |
| Elevated AST‡ | 2.2 % | 0.7 % |
No photosensitivity or vaginal candidiasis reported in controlled clinical trials (vs placebo)1
Study Design: The safety and efficacy of ORACEA Capsules in the treatment of inflammatory lesions (papules and pustules) of rosacea was evaluated in two randomized, placebo-controlled, multicentered, double-blind, 16-week Phase 3 trials involving 537 subjects (total of 269 subjects on ORACEA Capsules from the two trials) with rosacea (10 to 40 papules and pustules and two or fewer nodules)1
*30 mg immediate release and 10 mg delayed release beads
†The Investigator’s Global Assessment (IGA) is an evaluation tool measuring the severity of rosacea. IGA 4 refers to severe rosacea; IGA 3 describes moderate rosacea; IGA 2 is mild rosacea; IGA 1 is almost clear of rosacea, and IGA 0 is clear of rosacea
‡AST, aspartate aminotransferase
PATIENT ADHERENCE
ORACEA capsules DAILY DOSE MAY help patients stay ON course5
ORACEA Capsules with one daily dose, may improve patient adherence5,7
A simple treatment that fits into your patients’ morning routine7

A once-daily dose is convenient
and may promote patient adherence
to treatment5
A well-tolerated formulation1,9
- AEs did not include nausea, diarrhea or vomiting9
- Superior GI tolerability vs doxycycline 100 mg9
- No evidence of bacterial resistance in a 9-month study4
VISIBLE RESULTS
Just as powerful as doxycycline 100 mg9
ORACEA Capsules, with their modified release mechanism, are safe and
effective when treating the inflammatory lesions of rosacea, meaning higher-
dose doxycycline’s may not be needed1,9
The 30 mg immediate release and 10 mg delayed release mechanism makes ORACEA Capsules just as
powerful as doxycycline 100 mg, without crossing the antimicrobial threshold1–3,9,10
Choose ORACEA Capsules
for your papulopustular rosacea patients
A unique
formulation
Learn how this formulation of 30 mg immediate release and 10 mg delayed release beads, does not compromise on strength2,7,9
Superior GI
tolerability
See how ORACEA Capsules showed superior gastrointestinal (GI) tolerability vs doxycycline 100 mg and did not induce symptoms of nausea, diarrhea, or vomiting9

Suitable for
long-term use§
Learn how to limit the risk of antibiotic resistance with ORACEA Capsules, a non-antibiotic dose that remained below the antimicrobial threshold in a long term4-6,8,11§
§No evidence of bacterial resistance in a 9-month clinical study4
DESIGNED WITH PURPOSE
TREAT PAPULOPUSTULAR ROSACEA FROM THE INSIDE OUT1–3,9,10
-

Immediate-release doxycycline
Immediate-release doxycycline (50 mg/2 x 20 mg) is not bioequivalent to
ORACEA Capsules7 -

Delayed-release mechanism
With ORACEA Capsules, 30 mg of the dose is released immediately, and the
remaining 10 mg is designed to work through a delayed-release mechanism7,8
- These once-daily capsules have been uniquely designed to provide a long-term treatment of the inflammatory lesions of rosacea1–3,7,9,10
- Not only do ORACEA Capsules provide a powerful non-antibiotic dose of doxycycline, but one that keeps your patients safely below the antimicrobial threshold4–6,8,11
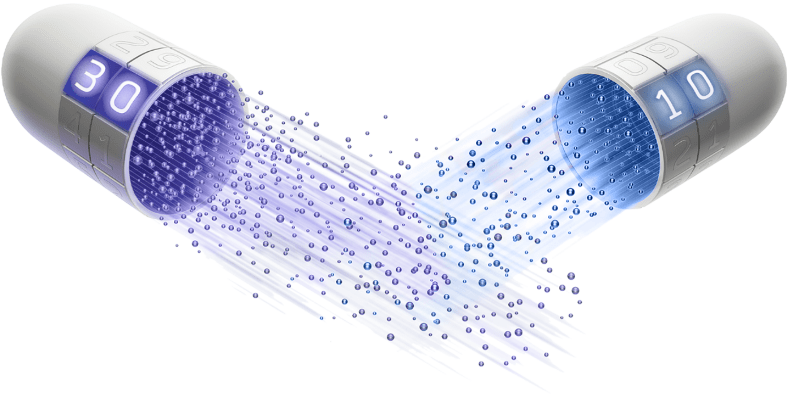
30 MG
IMMEDIATE RELEASE BEADS
are released in the stomach (pH 1.1)8
10 MG
DELAYED RELEASE BEADS
are released in the small intestine (pH 6)8
Image of capsule is a simulation, for illustration purposes only. Not the actual capsule
PRECISION CARE
The pharmacology of ORACEA Capsules
- The rate and extent of absorption of immediate release doxycycline (2 x 20 mg) is not equivalent to
ORACEA Capsules8 - The capsules are coated in a layer that immediately releases the 30 mg beads once it hits the
stomach (pH 1.1)8 - The remaining 10 mg beads dissolves when the capsule reaches the small intestine (pH 6)8
- The concentration of doxycycline is high enough to be efficacious, while remaining below
the antimicrobial threshold8
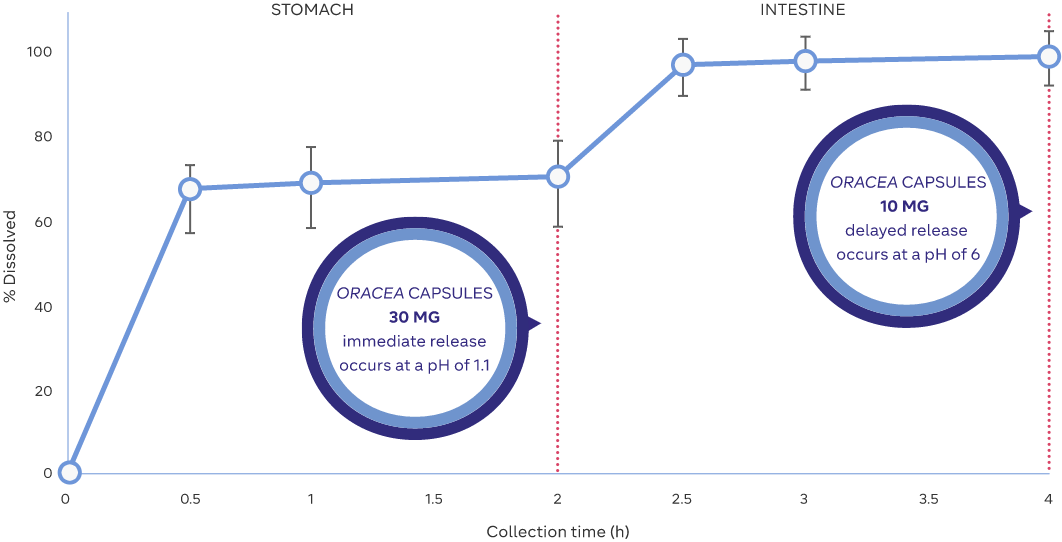
The line of the graph represents ORACEA Capsules in vitro controlled release, averaged from three batches
Adapted from: Data on file. ORACEA PK Profile. Galderma Laboratories, L.P
**IGA, Investigator’s Global Assessment
STAY CONNECTED
Sign up and
stay up-to-date
For the latest news, research, and insights into papulopustular rosacea, subscribe now! You’ll also receive patient resources and toolkits, helping you treat your patients throughout their rosacea journey
Important Safety Information
Indication: ORACEA® (doxycycline) 40 mg* capsules are indicated for the treatment of only inflammatory lesions (papules and pustules) of rosacea in adult patients. ORACEA does not lessen the facial redness caused by rosacea. Adverse Events: In controlled clinical studies, the most commonly reported adverse events (>2%) in subjects treated with ORACEA were nasopharyngitis, diarrhea, hypertension and sinusitis. Warnings/Precautions: ORACEA should not be used to treat or prevent infections. ORACEA should not be taken by patients who have a known hypersensitivity to doxycycline or other tetracyclines. ORACEA should not be taken during pregnancy, by nursing mothers, or during tooth development (up to the age of 8 years) and may cause reversible inhibition of bone growth. If Clostridium difficile associated diarrhea (CDAD) occurs, may need to discontinue ORACEA. Although photosensitivity was not observed in clinical trials, ORACEA patients should minimize or avoid exposure to natural or artificial sunlight. The efficacy of ORACEA treatment beyond 16 weeks and safety beyond 9 months have not been established.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
*30 mg immediate release and 10 mg delayed release beads
REFERENCES
1. Del Rosso JQ, et al. Two randomized phase Ill clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatmentof rosacea. J Am Acad Dermatol. 2007;56(5):791–802. 2. Bhatia N. ORACEA 40 mg capsules for papulopustular rosacea. The Dermatologist. 2013;6(21s):1-4. Available at: https://www.hmpgloballearningnetwork.com/site/thederm/site/cathlab/event/oracea-40-mg-capsules-papulopustular-rosacea-1. Last accessed: September 2021 3. Theobald K, et al. Anti-inflammatory dose doxycycline (40 mg controlled-release) confers maximum anti-inflammatory efficacy in rosacea. Skinmed. 2007;6(5):221–226. 4. Preshaw PM, et al. Modified-release sub-antimicrobial dose doxycycline enhances scaling and root planning in subjects with periodontal disease. J Periodontol. 2008;79(3):440–452. 5. Fowler JF. Anti-inflammatory dose doxycycline for the treatment of rosacea. Expert Rev Dermatol. 2007;2(5):523–531. 6. Baldwin HE. Diagnosis and treatment of rosacea: state of the art. J Drugs Dermatol. 2012;11(6):725–730. 7. ORACEA [package insert]. Fort Worth, TX: Galderma Laboratories, L.P.; 2014. 8. Data on file. ORACEA PK Profile. Galderma Laboratories, L.P. 9. Del Rosso JQ, et al. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7(8):573–576. 10. Wise RD. Submicrobial doxycycline and rosacea. Compr Ther. 2007;33(2):78–81. 11. Etchegaray JP, Wagner N, Shah MS, Difalco RJ, inventors; Galderma S.A., Cerovene, Inc. assignees. Doxycycline formulations, and methods of treating rosacea. US Patent 8,652,516 Bl. February 18, 2014






